iridium electron configuration|Iridium (Ir) : Tagatay The arrangement of electrons in iridium in specific rules in different orbits and orbitals is called the electron configuration of iridium. The electron configuration of iridium is [ Xe ] 4f 14 5d 7 6s 2 , if the electron arrangement is through orbitals. a brilliant swarm of light bleeding into the air - English Only forum A broken light bulb - English Only forum A candle is not diminished by giving another candle light. - English Only forum a cast iron light - English Only forum a certain angled light - English Only forum a chink of light - English Only forum a chink of light = a pencil of light ?
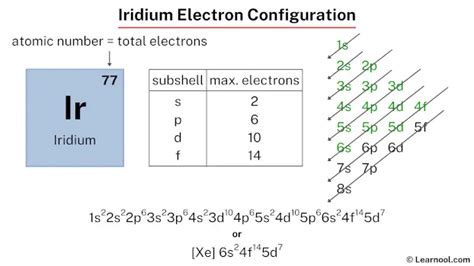
iridium electron configuration,The arrangement of electrons in iridium in specific rules in different orbits and orbitals is called the electron configuration of iridium. The electron configuration of iridium is [ Xe ] 4f 14 5d 7 6s 2 , if the electron arrangement is through orbitals.Learn the electron configuration of iridium, a rare and corrosion-resistant transition metal, and its main characteristics, precautions and applications. The web page also provides .Iridium is a transition metal with symbol Ir and atomic number 77. Its electron configuration is [Xe] 4f 14 5d 7 6s 2, with valence electrons of 2, 3, or 4.Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .Electronic configuration of the Iridium atom. Valence electrons. Orbital diagram.iridium electron configuration Iridium (Ir) Electron Configuration. [Xe] 4f 14 5d 7 6s 2. Iridium is the most corrosion-resistant metal known. Physical Properties. Aufbau principle. First, find electrons of iridium atom. Periodic table | Image: Learnool. The atomic number of iridium represents the total number of electrons of iridium. Since the atomic number of . The ground state electron configuration of ground state gaseous neutral iridium is [Xe].4f 14.5d 7.6s 2 and the term symbol is 4 F 9/2. Schematic electronic configuration of iridium. The Kossel shell .Iridium (Ir) ← Osmium. Platinum →. General Properties. Physical Properties. Media. States. Energy. Electrons & Oxidation. Nuclear. Isotopes. Mass Number. The sum of the .Technical data for Iridium. Click any property name to see plots of that property for all the elements. Notes on the properties of Iridium: Specific Heat: Value given for solid phase. .
Element Iridium (Ir), Group 9, Atomic Number 77, d-block, Mass 192.217. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the .Electron configuration 4f 14 5d 7 6s 2: Electrons per shell: 2, 8, 18, 32, 15, 2: . Iridium is a chemical element; it has symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum . Iridium atoms have 77 electrons and the shell structure is 2.8.18.32.15.2. The ground state electron configuration of ground state gaseous neutral iridium is [ Xe ]. 4f 14 . 5d 7 . 6s 2 and the term symbol .Abbreviated electronic configuration of Iridium. The ground state abbreviated electronic configuration of Neutral Iridium atom is [Xe] 4f14 5d7 6s2. The portion of Iridium configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Xe]. For atoms with many electrons, this notation can become lengthy and so an .
Iridium electron configuration, X-ray photoelectron spectra, and other elemental information – part of the XPS Reference Table of Elements. Hamburger Menu Button. Sign in. Don't have an account ? . Electron configuration: . A step-by-step description of how to write the electron configuration for Indium (In).In order to write the In electron configuration we first need to know t.Iridium (Ir) has an atomic mass of 77. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Electron Configuration [Xe] 4f 14 5d 7 6s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 7 6s 2: Orbital Diagram. Nuclear. Radioactive: No: Isotopes. SymbolThe number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Indium is [Kr] 4d10 5s2 5p1. Possible oxidation states are +3.The electron configuration of iridium (Ir) is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s² 4f¹⁴ 5d⁷.Iridium electron configuration, X-ray photoelectron spectra, and other elemental information – part of the XPS Reference Table of Elements. Hamburger Menu Button. Sign in. Don't have an account ? . Electron configuration: .Chemical element, Iridium, information from authoritative sources. Look up properties, history, uses, and more. . 2.2 Electron Configuration [Xe]6s 2 4f 14 5d 7. Los Alamos National Laboratory, U.S. Department of Energy. .
iridium electron configurationElectronic configuration of the Iridium atom in ascending order of the levels: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 7 6s 2 Reduced electronic configuration Ir: [Xe] 4f 14 5d 7 6s 2. Below is the electronic diagram of the Iridium atom Distribution of electrons over energy levels in the Ir atom
The electron configuration of iridium is much longer than aluminum. Although drawing out each orbital may prove to be helpful in determining unpaired electrons, it is very time consuming and often not as practical .
Iridium - Tableau Périodique - Numéro Atomique - Masse - Rayon - Densité. Par rapport à d'autres éléments, l'iridium a une structure et un rayon différents et a donc une masse et une densité atomiques différentes. . La configuration .

Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .

Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Iridium (Ir) Iridium - Properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more; Interactive periodic table of the chemical elements. . Electron Configuration [Xe] 4f 14 5d 7 6s 2. Ir. Iridium is the most corrosion-resistant metal known. Physical Properties. Phase. Solid. Density. 22.56 .
Electron configuration: [Xe]4f 14 5d 7 6s 2. Oxidation states: +3,4 Major edges What are these symbols? L3 11215. M4 2116. M5 2040. O2 63. O3 51. Minor edges. L1 13419. . Related elements: Iridium Oxygen. Data contributor: Gatan Beam energy (keV): 200 .Element Indium (In), Group 13, Atomic Number 49, p-block, Mass 114.818. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.
iridium electron configuration|Iridium (Ir)
PH0 · WebElements Periodic Table » Iridium » properties of
PH1 · Technical data for the element Iridium in the Periodic Table
PH2 · Iridium electron configuration
PH3 · Iridium (Ir)
PH4 · Iridium
PH5 · Electron configuration of Iridium
PH6 · Electron configuration for Iridium (element 77). Orbital diagram
PH7 · Complete Electron Configuration for Iridium (Ir)